LA dimension and volume have become vital parameters in recent times, especially, with the entity of HFpEF is becoming so common. LA not only acts as a live barometer, reflecting all that happens in LV, but it is also a chronic marker of LV diastolic function. (Funnily referred to as HBA1c of diastolic dysfunction)
What is normal LA dimension & volume ?
- Normal left atrial diameter < 4.1 cm in men or < 3.9 cm in women
- Normal left atrial volume indexed for body surface area (BSA) is 34 ml/m2 for both women and men
Which part of the cardiac cycle do we measure?
Ever since Wiggers introduced the overwhelming concept of LV systole and diastole, most of us ignored the fact that atria do have a separate contraction relaxation cycle, independent of what happens in the ventricle. Of course, atria and ventricles act as a single chamber in diastole. In reality, atria lack true boundaries when it acts as a conduit. The LA dimension varies considerably during the atrial cardiac cycle. Look at the LA pressure-volume loop, which can frighten anyone, with its horizontally lying figure of 8 pattern. During every cardiac cycle, the volume reaches atleast two troughs and one peak.

Don’t get frightened with this graph, spend some time, and you will get it right, Begin at “3” o clock position with the onset of diastole with a downsloping green loop, that continues as the red line of atrial contraction to end up in systole. The entire black loop, that happens during ventricular systole depicts the true reservoir function. with MV closed. ,
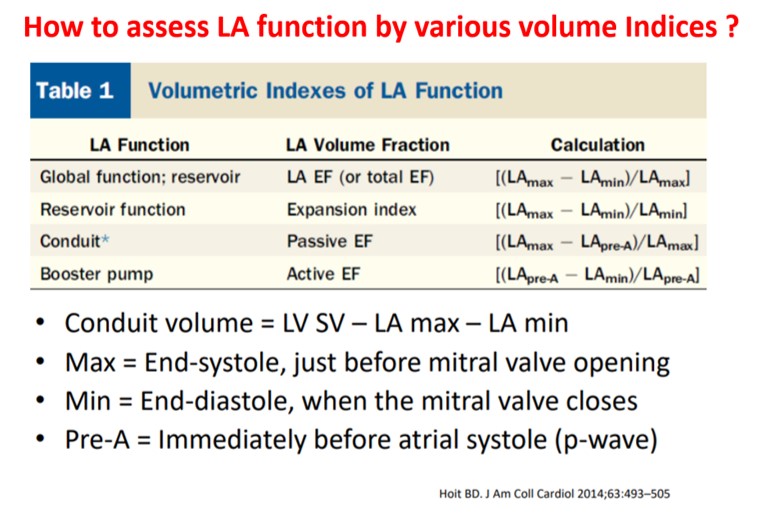
As of now, we have a consensus, LA volume is measured typically in LV end-systolic frame. ( Rather, we measure it at maximum LA volume ) However, we have 4 different LA volumetric components to assess, as this article excellently depicts. (Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014 Feb 18;63(6):493-505.)
What could be the limitations of the traditional end-systolic measurement?
No single measurement will give an overall LA function assessment. But still, Somehow, we have measured the maximum LA volume as a reference of true diastolic function. This happens in LV end-systolic point where atria reach the maximum size. But, here is a catch, we assess the left atrial function before its main physiological function of emptying takes place.
How about assessing LA efficiency after it completes its job, ie end diastole?
In LV function end-systolic dimension has pride of place as it is devoid of influence from loading condition. If applying the same logic, the “end atrial” systolic dimension(Which is the same as LV end-diastolic point/or post A ) should be perfect. It can also help measure the residual LA volume after its systole.
A potential advantage of LV end-diastolic dimension (The Heart & Soul study )
Maybe, this is less affected in the presence of MR systolic jet will spuriously elevate LA volume. In AF also this parameter is less likely to be influenced by LA preload.
Final message
Suddenly, we are debating a fundamental Issue, ie timing of LA measurement. While the end-systolic size/volume is the current standard, the LA dimension in the end diastole also provides useful info. There are at least 4 different LA volumes, at different parts of the LA cycle that need to be studied for a proper understanding of diastology (Unlike LV which has only two).
Now, we may need to ponder, if there is a mean LA volume, measured with the 3D volumetric analysis or MRI, that could be representative of the global LA function.
Reference