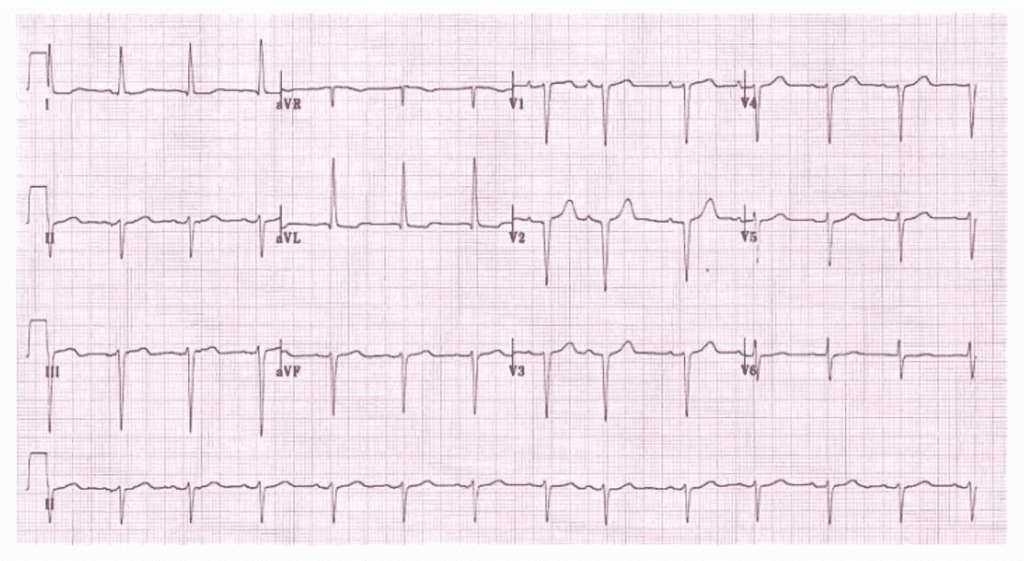
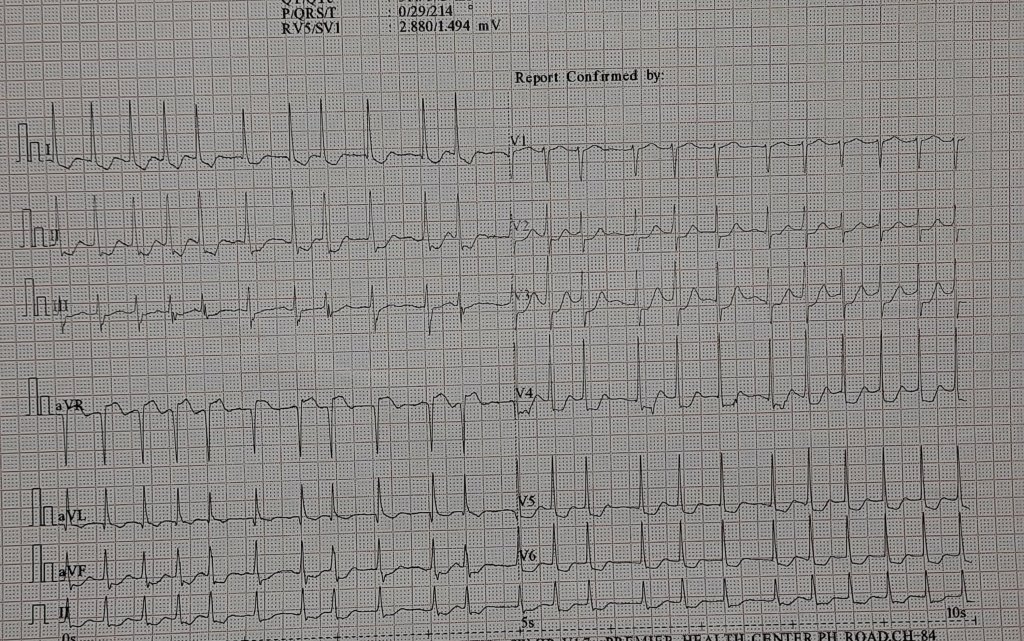
Can you believe that 68% of marathon runners show elevated Troponin levels after crossing the finish line? . 11% of them have significant levels that could lead to a diagnosis of ACS if they experience chest pain and end up in the hospital. (Fortescue EB 2007 )
Clinical experience suggest, that it doesn’t require a marathon race to bring troponins into the bloodstream. Any heavy, prolonged physical exertion can potentially release these biomarkers.

How much Troponins are released in these runners ? (Ref 3)
Most runners (68%) had some degree of troponin increase (troponin T > or = 0.01 ng/mL or troponin I > or = 0.1 ng/mL), and 55 (11%) had significant increases (troponin T > or = 0.075 ng/mL or troponin I > or = 0.5 ng/mL))
Troponin distribution within myocyte
Troponins are structural cardaic protiens. 90 % are attached to contractile elements. Free floating troponin in cytoplasm (myoplasm) are detected in about 6–8% for cTnT and 3.5% for cTnI
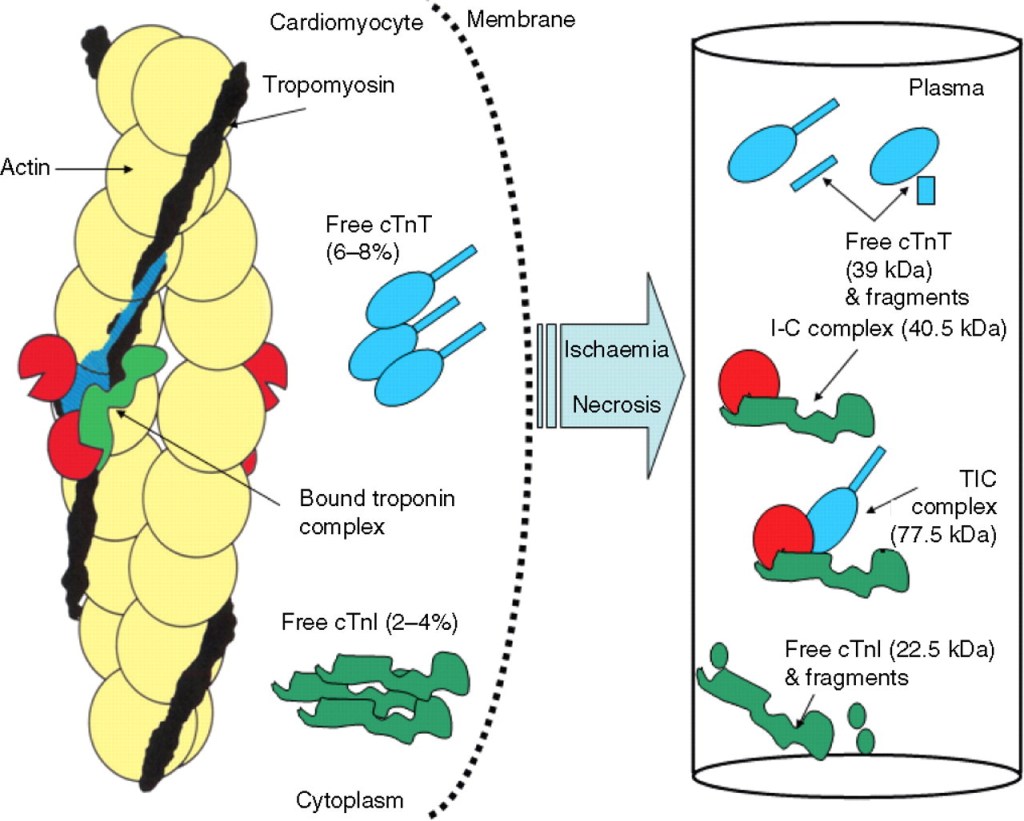
Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Annals of Clinical Biochemistry. 2008;45(4):349-355. doi:10.1258/acb.2007.00722
Mechanism of Troponin leak
Why should a hale and healthy person , (in fact a super normal humans) release cardaic Troponin into blood stream ?
The following are the putative mechanism mentioned in the best availabe literature.(Ref 1)
- An increased cardiomyocyte sarcolemmal permeability attributable to cell wounds,
- Release of extracellular blebs
- Increased exocytosis rates can be considered as reversible cardiac damage,
- Physiological increase of cardiac troponin concentrations.
- Similarly, an increased cardiomyocyte turnover may transiently increase cardiac troponin concentrations.
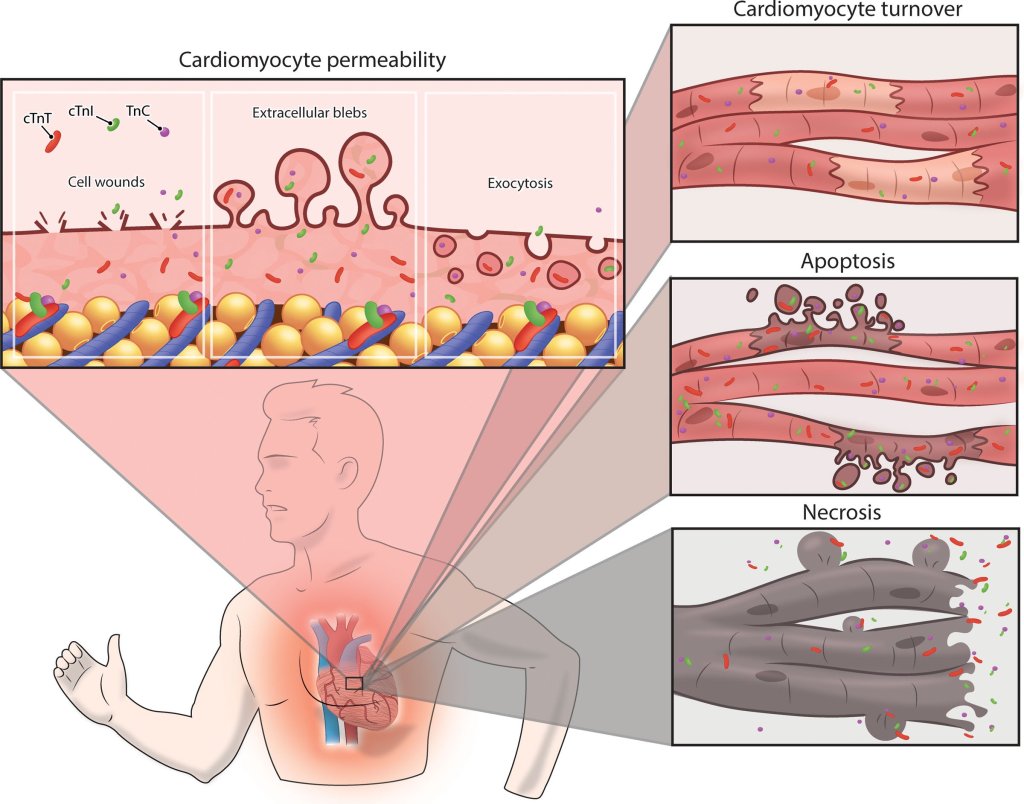
(Image courtesey & source : Aengevaeren VL Circualtion 2021)
Does any of the reasons given above appear convincing ?
What is more likely is that some unknown mechanical stretch and strain somehow fatigues the sarcolemmal cell membrane, and the cytosolic free Troponin T and I gets leaked across. In all likelihood, it does not imply myocardial necrosis, i.e., damage to structural proteins (Opinions are divided, still, some claim it does happen (Ref 1)
How does skeletal muscle behave during long distance running ?
Intense, unaccustomed systemic exercise increases myoglobinuria and rhabdomyolysis (Ref 2). It’s no surprise that the heart also excretes Troponin locally in a similar fashion.
How to diagnose ACS in these runners ?
Only clinical and ECG and follow up.
Long term consequnece of Troponin release in these atheletes
None in most. The apparently leaky membrane heals and settles down . However (Ref 1) do share some evidence for long term sequale in few . Who are those few & how to identify them ? . No answers as yet.
Final message
Troponins are “dangerously funny” molecules, that can either be a sure shot marker a heart attack or simply appear in an absolutely healthy person and laugh at you. This is a classic example, clinical acumen and examination can never become obsolete in any technological era.
An ethical & legal offshoot
Wish, this nebulous nature of biomarkers should teach some important lessons to the ever-hungry litigation specialists, the esteemed medical juries, as well as to beloved patients. Request them to show some sympathy for the cardiologists who grapple with multiple uncertainties at odd hours.
It is unavoidable, we may err in the “scientific guess game” played with Troponins .Some times, we are compelled to admit normal persons in CCU, for suspected ACS with borderline elevation of these biomarkers. Missing an ACS also can happen, if Troponins play hide and seek when their releases are pulsatile. Apart from this, there is well known mismatch of Troponin , with its electrical counterpart ie, ECG. which can be as dynamic as it is.
Reference
A simple quiz for the fellows