Prologue
This happened some 20 years ago. One of my senior surgical colleague casually asked a Innocuous question to me, when I was in the elevator to reach my ward. “What do you guys really mean by LV dysfunction?” When I looked at him little amused, he said…“I am asking it seriously.” My fellow mumbled to me. that this query is apparently related to the echo report, we gave, few days ago, regarding a patient with AR and severe LV dysfunction .(who is posted for AVR the next week) . I gradually realized the gravity of the situation and question.
The ambiguity is the other name for LV dysfunction
The term LV dysfunction , we use umpteen times a day, simply convey a meaning, that LV is not working well. Is that right? What is it due ? It can be simple wear and tear, fatigue, myocyte damage, ischemic or non-ischemic, myocyte necrosis, death or apoptosis. Beyond that (recall myocytes form only 33% of LV mass, the rest are something else!) Non-myocytic interstitial infiltration, fibrotic, non-fibrotic, scarring, proteo-stasis, neo-cell proliferation, chronic organized myocardial edema. Apart from this, the now outmoded terms like hibernating and stunned myocytes are also included in that LV dysfunction basket.(Finally, don’t forget about hemodynamic afterload mismatch & dysfunctional diastology )
My surgeon friend was right after all. Which LV dysfunction are we talking about? Learned a harsh lesson. Our academic ignorance is explicit, still going around the wards majestically. Realised as a cardiologist we have the responsibility to find , (or at least make an effort) the various components of LV dysfunction.
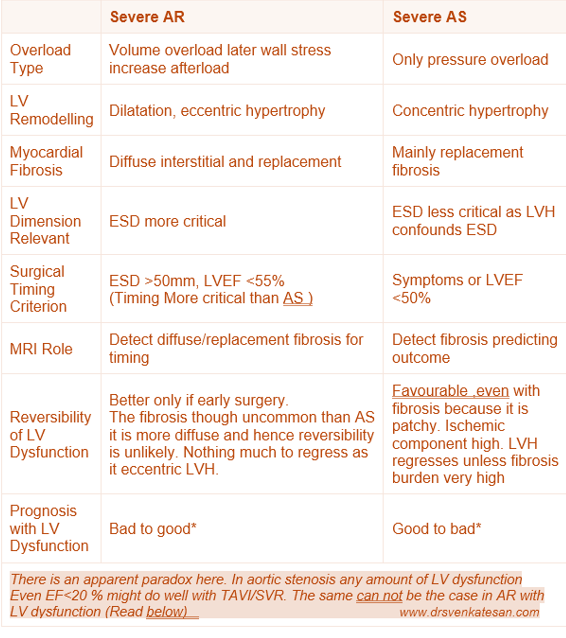
Mechanism of LV Dysfunction in AS vs AR
Differences in Mechanism
AR involves volume overload with LV dilation and eccentric hypertrophy with diffuse myocardial fibrosis. AS involves pressure overload with concentric hypertrophy and more focal replacement fibrosis. Both conditions lead to fibrosis, but the pattern, timing, and extent vary.
Clinical Implications in Timing of Surgery
Surgery in severe AR should be considered before irreversible LV dysfunction, guided by indexed LV dimensions (especially end-systolic diameter >50 mm or indexed >25 mm/m²), or LVEF <55%. Waiting too long allows fibrosis and remodeling to reduce recovery chances. In severe AS, surgery is indicated once symptoms develop or LV ejection fraction falls below 50%.
Role of LV Dimensions EDD and ESD
LV End-Diastolic Diameter (EDD): Reflects volume status and remodeling, important for AR where volume overload is predominant.It is less useful as it is pre-load dependent.
LV End-Systolic Diameter (ESD): ESD is unique parameter as it represent a topmost point ( north west) in cardiac pressure volume loop , when the contractility is load independent . it is a strong predictor of contractile function and prognosis.
Role of Cardiac MRI in Identifying Reversible LV Dysfunction
Role of MRI is vital. Cardiac MRI uses late gadolinium enhancement (LGE) to detect replacement fibrosis (scar) and T1 mapping/extracellular volume (ECV) quantification to detect diffuse interstitial fibrosis. *LGE: Late mean 20 minutes , the tissue stagnates and fails to get wash off and appear enhanced
Reversibility of LV Dysfunction in AS & AR : Is there a paradox ?
Patients with severe AR generally exhibit better reversibility and prognosis post-surgery compared to those with AS, (Ref 7 : This study found patients AR tend to have more diffuse fibrosis, which exhibits better regression after valve surgery compared to the focal replacement fibrosis in pressure overload conditions (like AS). This suggests better reversibility of LV dysfunction in AR) This finding is an apparent paradox, since we think LVH is more likely to have fibrosis.
Timing of surgery is more critical in AS or AR ?
- Patients with severe AS and very low LVEF can still experience significant improvement in LV function and survival after AVR.
- The explanation is that in AS, the primary problem is a mechanical pressure overload due to valve obstruction. AVR abruptly relieves the afterload, decreasing LV wall stress, and allowing recovery of myocardial function, sometimes dramatically.
- Even patients with severely reduced systolic function can see meaningful functional recovery post-AVR if myocardial fibrosis and irreversible damage are not advanced.
AR Patients with Moderate or Low EF After AVR
- In contrast, patients with AR who have even moderate reductions in LVEF tend to have worse outcomes post-surgery.
- The explanation lies in the gradual volume overload and progressive LV dilation in AR, leading to more diffuse myocardial remodeling and fibrosis that may be less reversible.
- The reduction in afterload after surgery in AR is more gradual and less dramatic than in AS, and by the time EF is moderately reduced, irreversible myocardial damage often limits recovery.
- Thus, surgery is ideally timed much earlier in AR (before moderate EF decline) to optimize
The non-forbidden question
Why should we wait? Is time a muscle only in ACS? Not in valvular heart disease? Can’t we intervene in all patients with severe AS/AR irrespective of LV function before it worsens?
Yes, time is indeed muscle even in VHD. This concept looks attractive. Many centers follow this, ignoring the current guidelines. (The issue here is dependence on artificial valves for the rest of life and the attendant risks.)
Final message
It is indeed true, cardiologists use the term LV dysfunction so commonly and casually, without elaborating on its true meaning. Whenever and wherever possible, we must take efforts to list and quantify various components of LV dysfunction, and also the likelihood of reversibility.
Reference
- Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, et al . Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc Imaging. 2017 Nov;10(11):1320-1333. doi: 10.1016/j.jcmg.2016.10.007. Epub 2016 Dec 21. PMID: 28017384; PMCID: PMC5683736.
- Pires LT, Rosa VEE, Morais TC, Bello JHSM, . Postoperative myocardial fibrosis assessment in aortic valvular heart diseases-a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2023 Jun 21;24(7