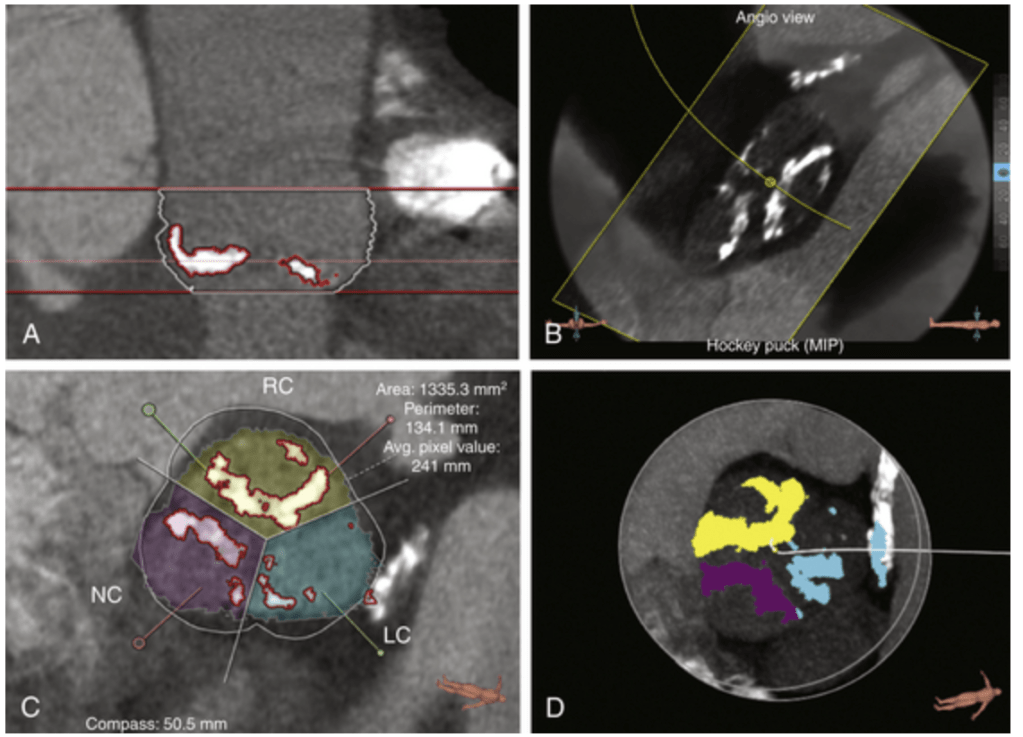
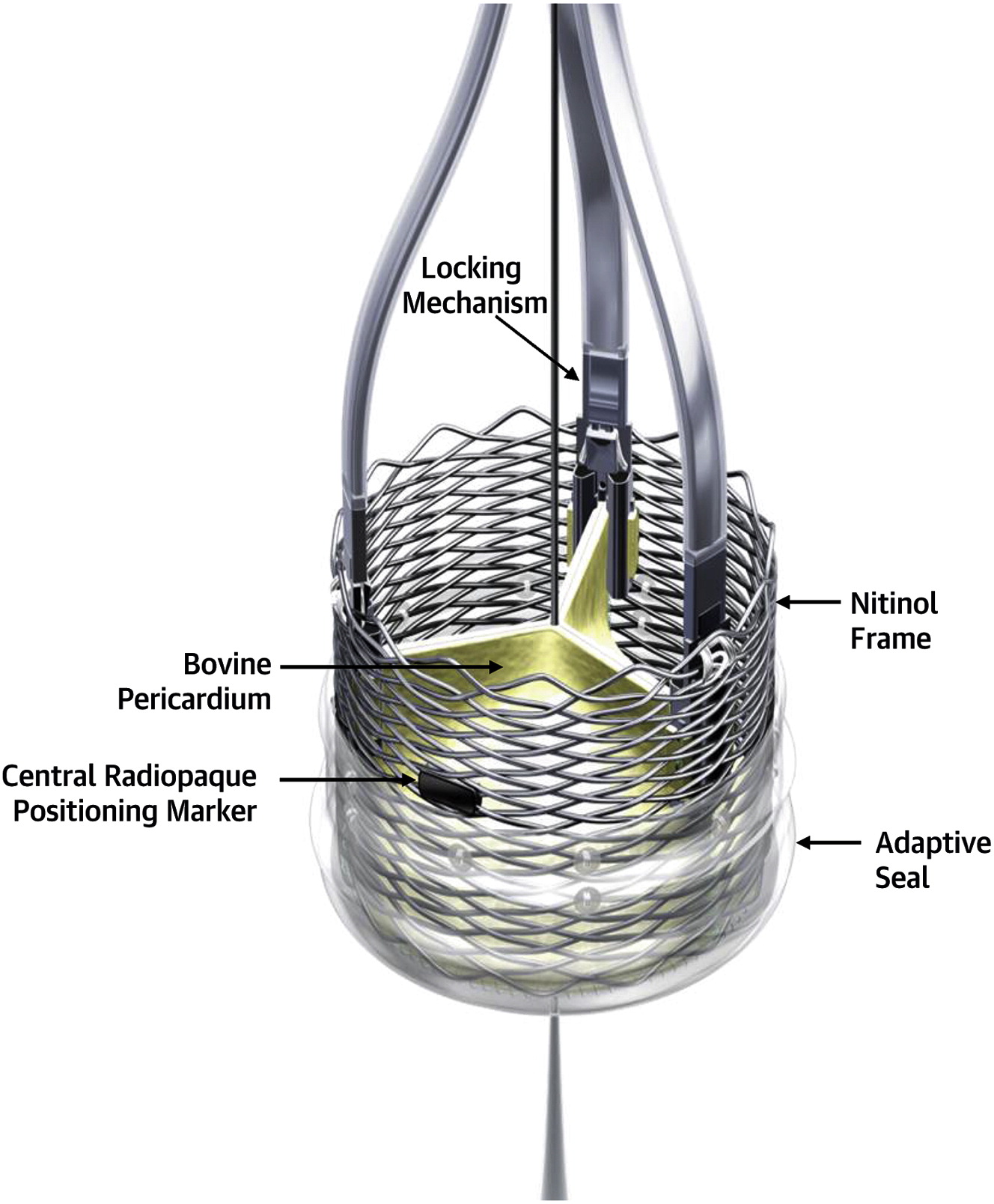
Innovation and Interventional cardiology are like Inseparable conjoined kids. When a mind blowing idea shakes hands with unprecedented technology, supported by an accommodating industry, such breakthroughs happen.
First In human : Creation of neo-coronary ostium & by pass stent to LAD
Here is an ultimate intervention, called the VECTOR procedure, that creates a tunneling pathway outside the regular anatomical pathways, like laying a road or metro tunnel under a mountain or river. (Ref 1 Circulation Cardiovascular Intervention 2026)
Combining the expertise of the retrograde approach of CTO and covered stent technology, a neo coronary ostium and an artificial artery is created that connects the ascending aorta and LAD, burrowing through the epicardium, myocardium, and pericardial plane.
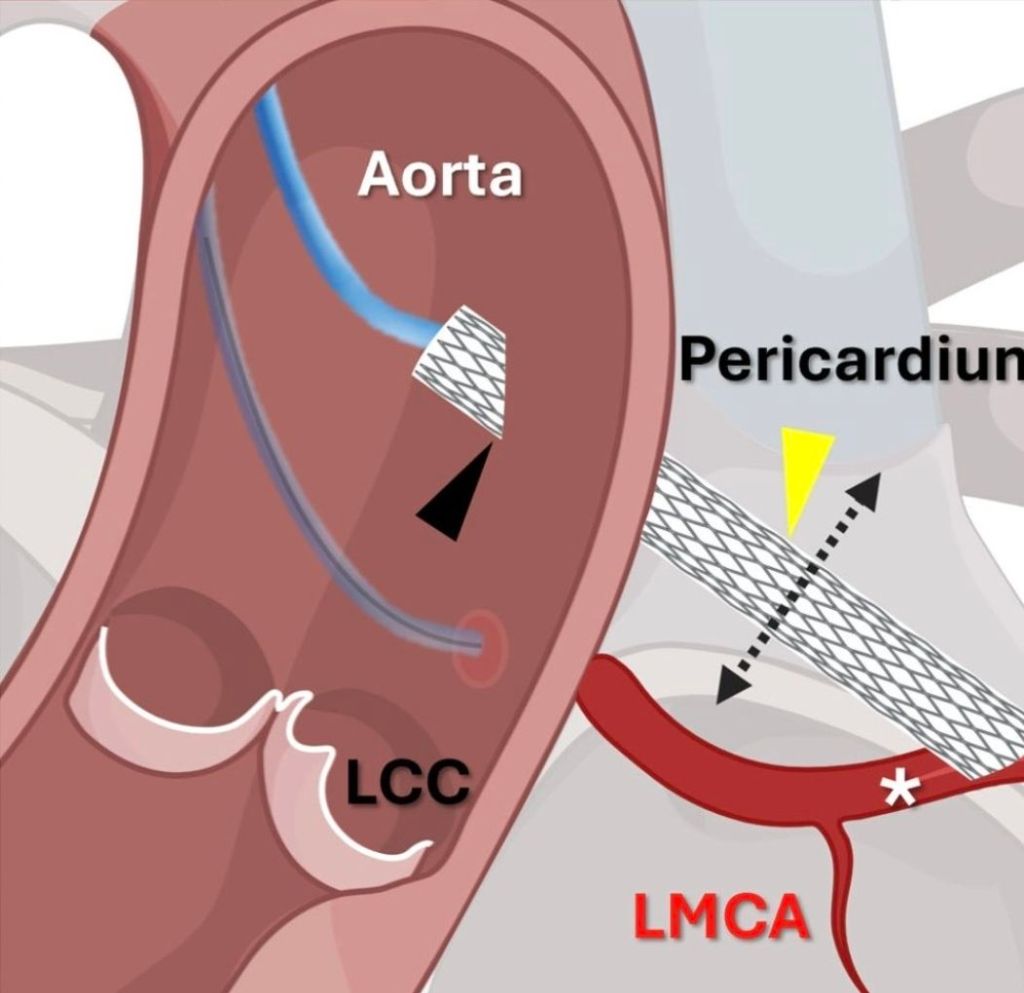
VECTOR procedure : Ventriculo-coronary transcatheter outward navigation and reentry (VECTOR)
1.Who is the patient ?
A very high risk post TAVR, who had a blocked his left coronary ostium. VECTOR was attempted in lieu of CABG, which probably he was not eligible.
2.Where it was done ?
I think it was done at Emory , the same place Andreas Gruentzig, show cased his magnum opus.
(It is a combined innovation from four institutes Structural Heart and Valve Center, Emory University Hospital, Atlanta, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, St Francis Hospital and Heart Center, NY, MedStar Washington Hospital Center and Georgetown University, Washington, DC0
3.What are possible percutaneous solutions to prevent coronary ostial obstruction ?
1.BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction)
2.UNICORN (Undermining Iatrogenic Coronary Obstruction With Radiofrequency Needle)
3.CATHEDRAL (CATHeter Electrosurgical Debulking and RemovAL)
The VECTOR procedure , first in human human was attempted
4.Now, what are challenges ? Will such procedure stand the test of time ?
The key question is, (apart from complexities and complications of the VECTOR procedure) how the covered stent will seal the peri-anastomotic site both in the aorta and coronary end?
5.Is this procedure possible in a non-TAVR situation in complex ostial and left main disease?
Time will tell
6.A venous and hepatic analogy
I don’t know this comparison is correct. VECTOR procedure has some similarities with the TIPS procedure done as percutaneous portal vein to IVC connection through the hepatic tissue planes (Vignali C, TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005 )
Final message
If we can, somehow make the procedure simple with smaller hardware, circumventing VECTOR will be an ultimate victory for mankind and sure to stand tall in the pinnacles of glory.
However , when adopting new technologies “Always be ready, to sacrifice science , if you think it would interfere with patient well being”.
Reference