One of my colleagues was joking ,nowadays patients force us to read or even educate us . I completely concurred with him.
Doctor, do you have any investigation to know how much is the plaque burden in my coronary artery?
Yes. Sure , we have many modalities, right from CT calcium , OCT, IVUS, NIR, etc.
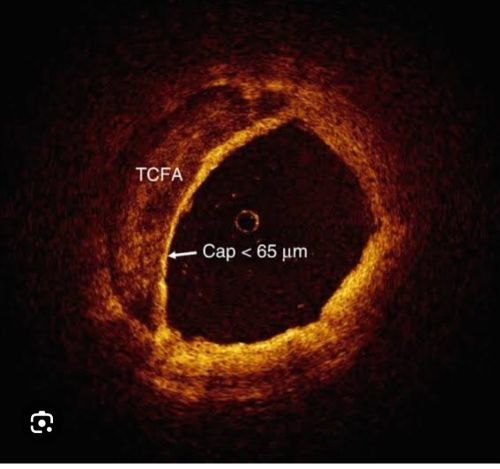
How would I know , which one of them is high risk and vulnerable ? I recently read in Forbes Sunday health supplement, It says ,it is better to know the thickness of the cap covering the plaque. It further says, about the risks of having a thin cap .(TCFA-Thin cap fibro atheroma.) Can you prescribe some tests to find out the thickness of these caps doctor ?
There you go, into the perils of hyper- education of modern society. So, you want me to teach you the molecular biology of Atherosclerosis ,right ?
Yes, now cardiologists and basic scientists are armed with unprecedented tools of intra-coronary imaging, like OCT, IVUS, NIR spectroscopy, etc. to decode the histological, biochemical, pathological secrets within the atherosclerotic plaques. Atherosclerosis is not a worrisome issue as long as it is contained deep within the vessel wall and doesn’t encroach the lumen. However, plaques, which are close to the lumen, are more prone to empty its contents into the lumen by a fissure or rupture triggering an event. In that sense, you have a reason to worry about TCA. Itis very much logical to expect the cap hat covers the plaque to protects and reduce the risk of its rupture.
This was a very attractive concept and we were able to analyse it with intracoronary optical coherence light-based imaging. For some time we really felt TCFA is really a marker of vulnerability. Now, we realize the concept of TCFA, has a thin layer of truth intertwined in a thick layer of myth .(Lee KY, Chang K. Understanding Vulnerable Plaques: Current Status and Future Directions. Korean Circ J. 2019 Dec;49(12):1115-1122. doi: 10.4070/kcj.2019.0211. )
In physiological state ,it is amazing to note, how a single layer of endothelial cell is able to withstand a lifetime of vascular stress. A plaque is covered by a thin cap that includes a endothelium (In tact in many plaque) While this layer is good enough to protect exudation of its content irrespective of the nature of lacquer content. A contrasting corollary is , a much thicker Aorta dissects without even a plaque if the pressure injury is more. TCFA are stressed from both abluminal and luminal aspect. Does it balance these forces ?
TCFA are defined as fibrous caps with less than 65 microns . The importance of this thickness is being questioned for two reasons now.
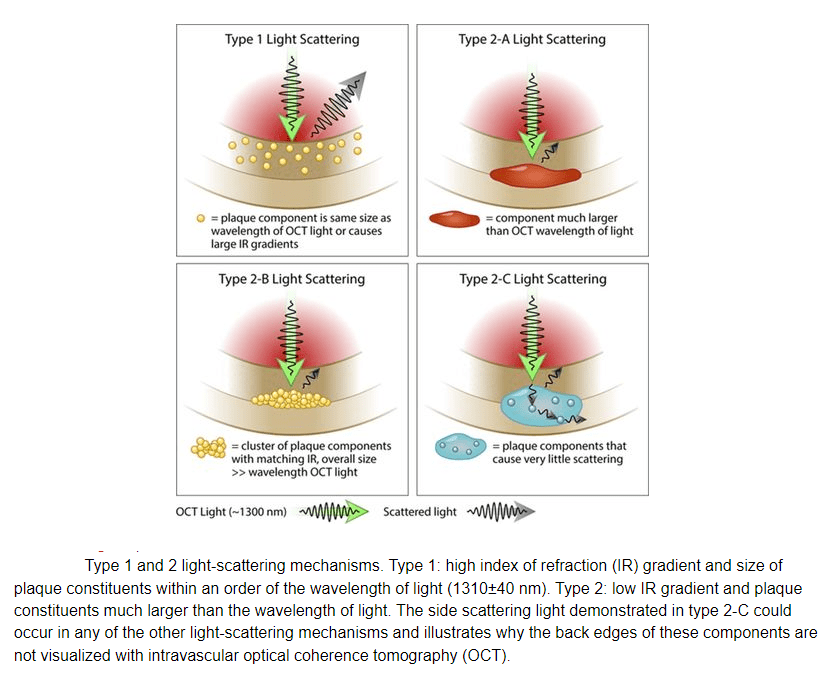
1,Technoloigical limitations of measuring cap thickness : False positive thin caps , false positive thick caps are equally common.
Factors influencing cap thickness
1.Superficial macrophages, ,
2.Loose connective tissue,
3.Punctate foam cells, and
4.Smooth muscle cell infiltration of the fibrous cap
5.Tangential light dropout(Ref 2)
2.The second one is a more fundamental flaw in our understanding. TCFC, after all could be just as innocent as any other plaque. This is based on the observation many of the thin capped plaques remain indolent while thick capped become a culprit . Cap thickness is just one of potential parameter of vulnerability, but many others known and unknown factors and forces operate on the plaque and make this TCFA simply irrelevant.
Some queries for advanced readers
What are the chances of a Thick cap fibro atheroma (ThCFA) getting ruptured ?
65 micron is not God prescribed cut off point . A simple adrenaline surge and eccentric plaque shoulder can be destabilized. A sudden shearing stress of intracoronary hypertensive spike can crack a 650 micron cap. On the contrary, even if the cap is thin , but the content it holds are non-necrotic , rupture is rare event and vice versa.
What are biological events that follow the rupture ?
Not every rupture leads to an ACS. The highly armed anti-coagulant and anti fibrinolytic system has enough resources to clear of the contents.(Unless it is over-whelmed ,which can be called as an act of God or a response to our past deeds)
Relationship between CT calcium score and TCFA
It is highly plausible, high calcium in plaques indicate, most caps are thickened and hardened. Though calcium score is a popular estimate of plaque burden, it is still a weak link to our ignorance and knowledge towards prevention of cardiac events.
Does TCFA prone for more coronary erosion ?
Though logic would suggest so, it appears , there is no meaningful relationship between them.
Does statins increase Thickness of TCFA ?
Yes. They are claimed in many studies. This is important, all patients need to take Statin to stabiles the plaque overall (Takarada wt al. Atherosclerosis. 2009 Feb;202(2):491-7.) More recently PCSK blockers Alirocumab was shown to increase TCFA at lesion levels study by NIRS (PACMAN-AMI study)
Final message
Coming to the question raised by the patient . My frank reply would be
“Never worry my dear patient total plaque burden or calcium scores. I would say, even the fear-mongering with blood levels of lipid sub-fraction, is an exaggerated state of pathological health consciousness. Life is short .We have better things to accomplish. The least we can bother in our life, is how to increase the thickness TCFA. Even if we find the total numbers of TCFA, what are we going to do with that info? My guess is ,the propensity of TCFA to damage your heart is 1000-fold less than the anxiety (associated with it) eroding some other innocuous plaque .
The chances of plaque misbehavior is as unpredictable as, the probability of a scale 4 Richter quake striking and shaking the basement of your house. So, instead of worrying about these TCFAs, concentrate on your work, about your lifestyle, stress, eating habits, and stop reading too much from all these health supplements. After all, please remember much greater men like Abraham Lincoln and Mahatma Gandhi lived a stellar life , in the grand old era when no one knew what was cholesterol and lipids.”
Reference