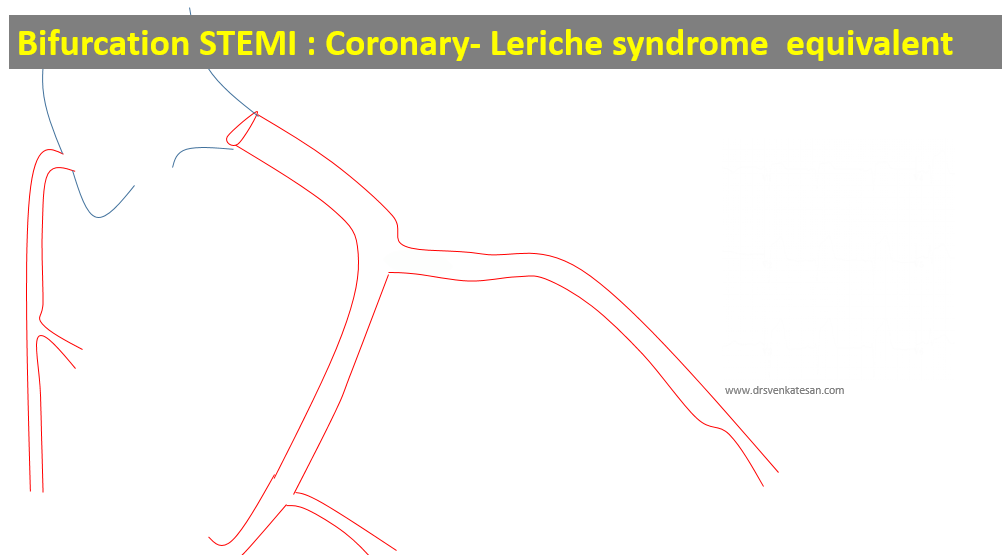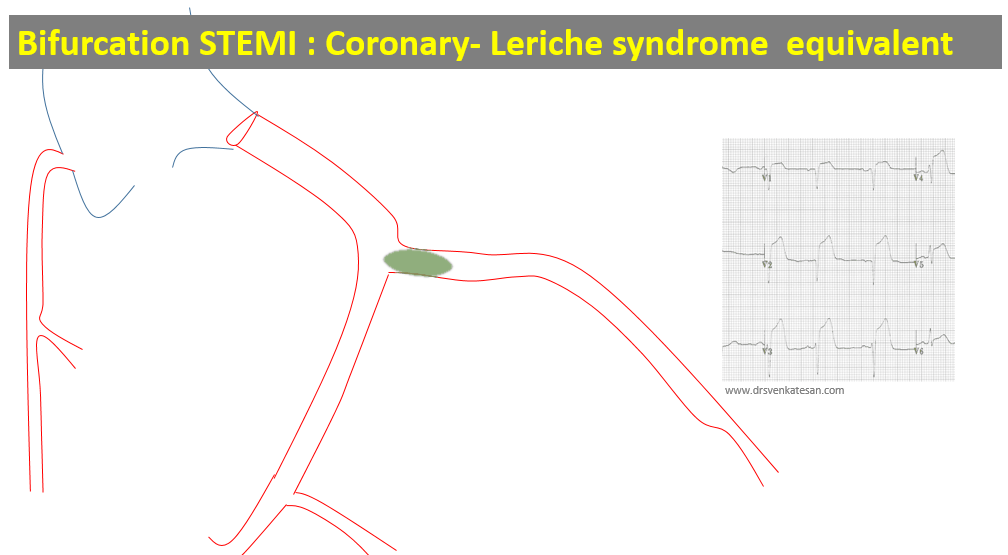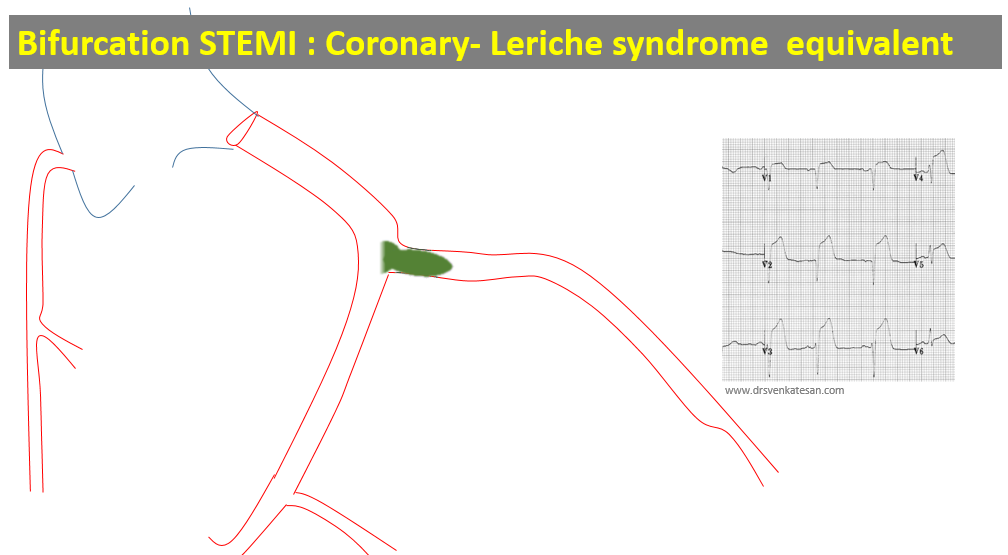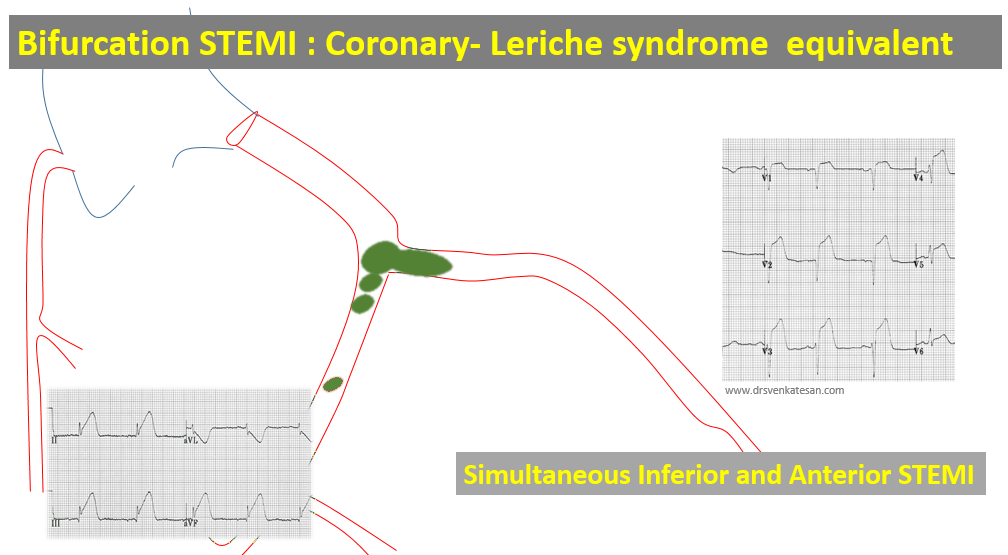
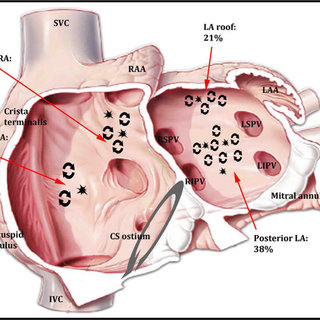
Like coronary blood flow, intra-cardiac electricity must flow in a pre-designated path at a specific time interval with absolute discipline. Any deviation or trespassing results in arrhythmia. While, minor aberrations are accepted, major deviations due to structural or functional reentry within the ventricles presenting as VTs (Scars, Infiltrates, etc) need immediate or at least early attention.
The term, VT mapping has been in vogue in clinical electrophysiology for more than half a century, right from Dr.Josephson and Wellens’ days. While , treating VTs with drugs is still a choice, permanent solutions by defining the VT circuit and ablating them, is the new norm. However , the difficulty is, demarcation of the VT circuit is still a tough job, especially since the VT circuit plays a mysterious hide-and-seek game during diastole. The current challenge is to draw the complete blueprint of the VT, especially the diastolic VT circuit.
The tracts that carry the diastolic electrical flow are located sub-endocardially, sub-epicardially , over right ventricular aspect, or finally through the ubiquitous concealed intramural paths.
Unless, we eliminate the entire circumference of the circuit the chances of recurrence is higher (This is contrary to the past belief that a one-time interruption of the VT circuit at some point of the circumference was considered good enough. (This is what DC shocks do for temporary reversion to sinus rhythm )
How to localize the diastolic pathways?
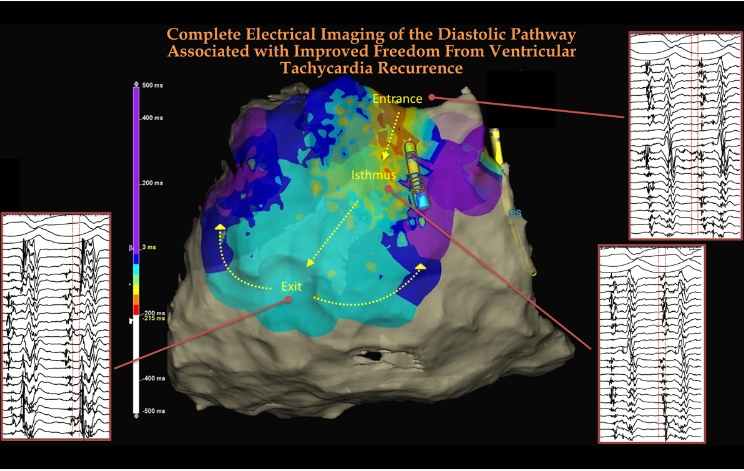
We must thank the technological innovators in the electro-anatomical mapping system, who are continuously upgrading and providing the best to us. The following image and video clip is one such demonstration of ablating hidden diastolic paths between the entry and exit points.
Diastolic blind spots between the entry & exit points of VT can be deep & dark
The final message
It’s very clear, that I can never be able to understand the technology and nuances behind the mapping and ablation. But, just wanted to share a simple thought with the general cardiologists after going through the above article. Like hemodynamics of blood , an “electro-dynamic” flow cycle exists that is critically important both in physiology and pathology . The learning point is that, in VT ablation, looking for anatomical diastolic tracts and its electrical activity becomes a key exercise.
How can we remember this EP lesson easily ?
We can take a cue from the vintage clinical auscultation classes, where we ask the medical students to look for MDM (mid-diastolic murmur) in mitral stenosis in the left lateral posture in expiratory phase. Now in modern electro-physiology, it is time to teach young cardiology fellows a new rule of thumb, always look for the (mid )diastolic electrical flow in any scar-induced VT.
Reference
Alexios Hadjis , Antonio Frontera. Luca Rosario Limite , et al Complete Electroanatomic Imaging of the Diastolic Pathway Is Associated With Improved Freedom From Ventricular Tachycardia Recurrence Circ Arrhythm Electrophysiol. 2020;13:e008651. DOI: 10.1161/CIRCEP.120.008651
Next question in the queue
Can a VT occur without an exit point ? (I have been looking for a long time for an answer)