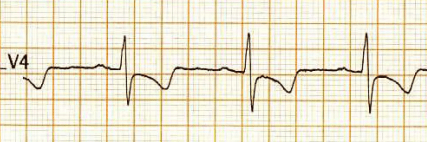
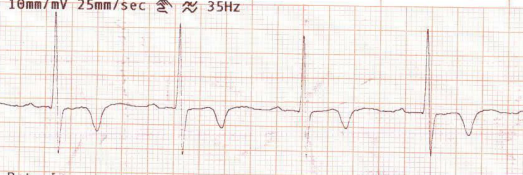
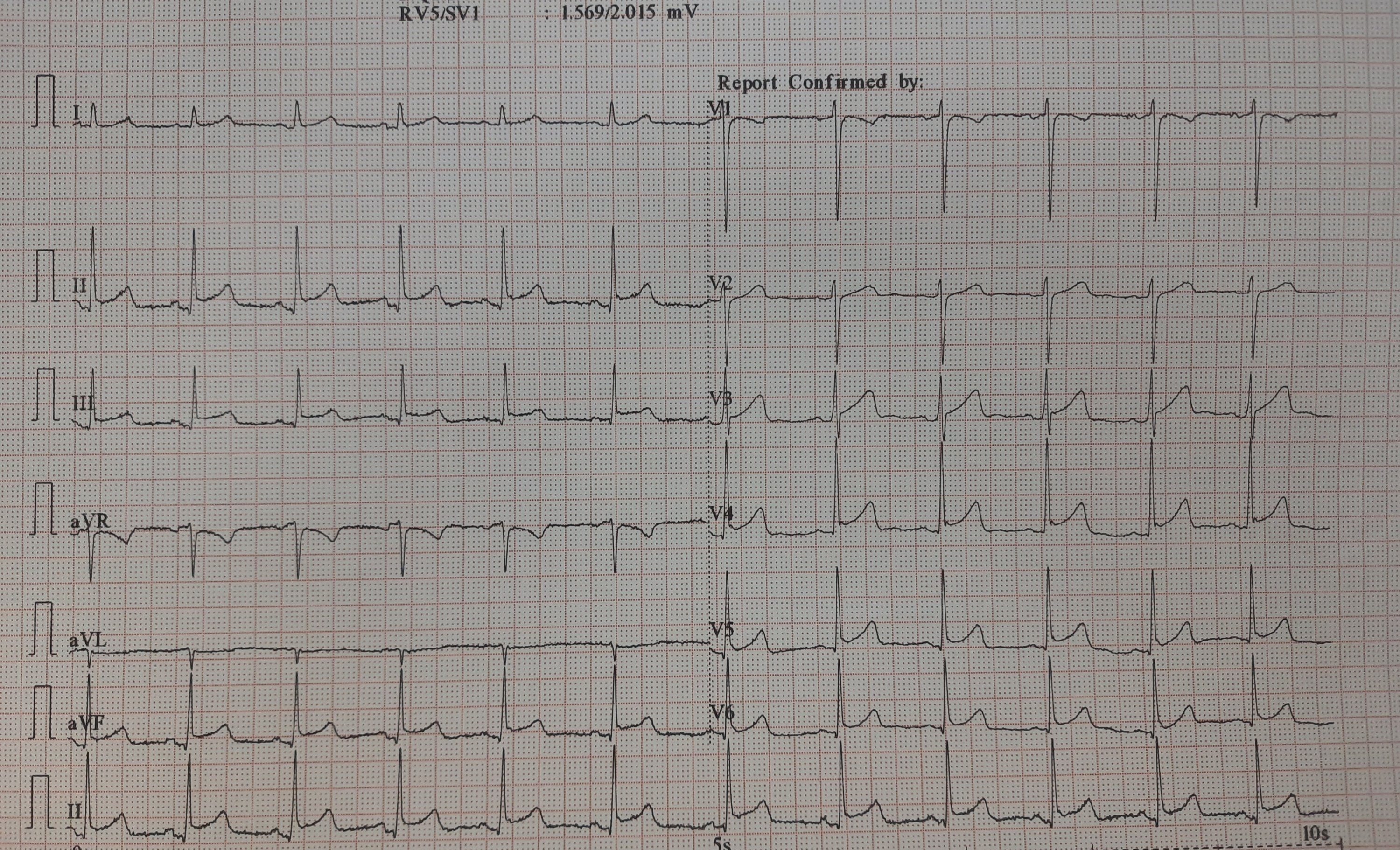
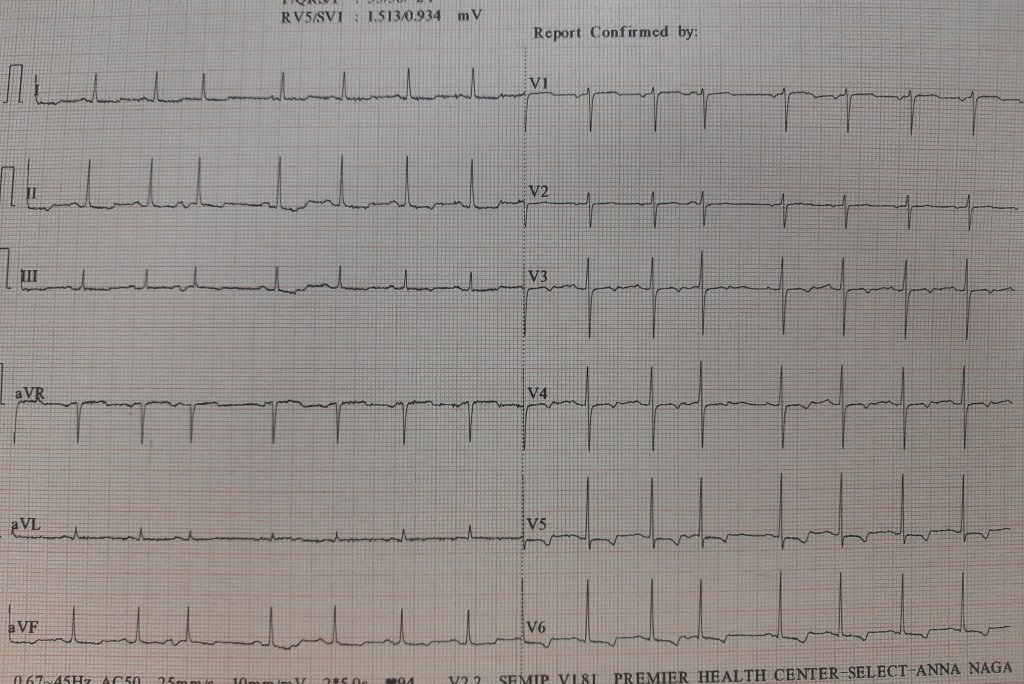
This is the Incidental ECG of an apparently healthy 50-year-old businessman, recorded while applying for health insurance
How will you describe this ECG?
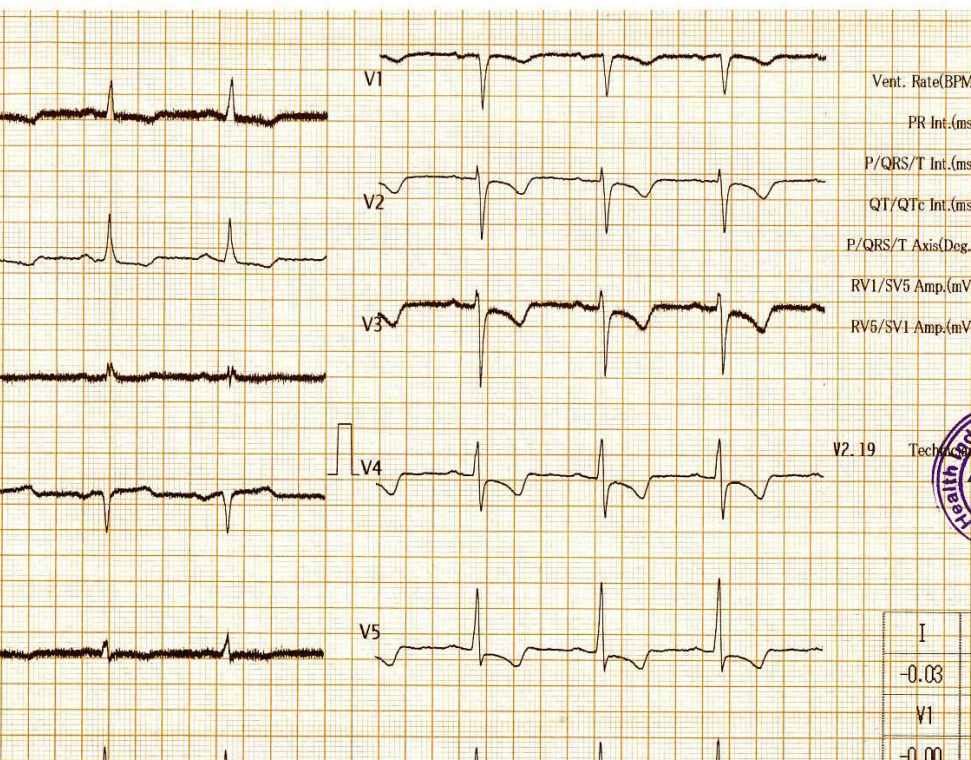
Why this big fuss about this ECG?
Such ECGs are so common. Looking at the ST segment, we are supposed to think of significant CAD,, LVH, Aortic stenosis or variants of cardiomyopathy, and sometimes electrolytic shifts. The fact that it is recorded at rest, and the patient is absolutely asymptomatic, it is very unlikely there is ongoing ischemia.It could be a myocardial origin or an unknown repolarisation pattern. But, one thing is clear, we can’t send this guy under the label of non-specific ST/T changes.
The Echo was done it was normal. No WMA, LVH. The aortic valve was perfect.
Is CAG indicated here?
Three responses came from three different cardiologists. Everyone agreed, the stress test is not going to be useful, as baseline is unstable
- Absolutely not Indicated, since he is asymptomatic. I believe the history and Echo. Please follow him up
- A definite yes for CAG. (Being a scientific cardiologist, without excluding CAD, I can’t be at peace. Will do at least a CT angiogram)
- A third cardiologist said a CT angiogram is waste of time and wanted to do a radial CAG in 10 minutes in his newly opened hi-fi radial lounge.
What happened then?
Don’t know, whether he underwent CAG or not. But, I can confidently say he will have a normal coronary angiogram. How can you be so confident? Confidence doesn’t mean I am correct. Look at the ST segment again. It is not true ischemic depression. It is neither non-sustained nor horizontal or downsloping*, This could be referred to as, primary T inversion with secondary ST segment dragging. Regarding the management, the first response is ideal,
*Classical slope should begin at J point. Late downsloping has little predictive value as in this ECG.
Is Echo good enough to rule out structural heart disease?
Even after the echo was reported normal, few questioned the quality of the echocardiogram and asked to look specifically for apical wall motion with speckle track and GLS. ( I know, MRI is a must nowadays to rule out structural heart disease as Echo can’t rule out intrinsic myocardial disarray, infiltration, etc)
How is ST dragging different from ST depression?
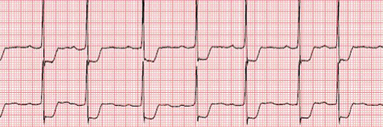
Classical horizontal ST depression
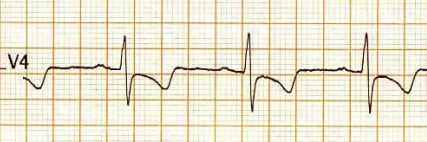
ST dragging due to T wave pulling
For ST depression to happen, the junction point needs to be depressed. Note here junction is held high, only the distal half of the ST segment dips rather dragged by the phase 3 repolarization process which corresponds to the proximal limb of T waves. Our observation is, this type of ST drag is less sinister and usually indicates a myocardial ST defect, rather than an ischemic one. (Examples: concealed LVH or forme fruste of HCM or Infilitrative cardiomyopathy-related entities.)
Final message
The purpose of posting this ECG is, some ST segments create disproportionate panic than it deserves. The concept of T waves pulling down the distal part of the ST segment which can be called ST segment dragging is being proposed here.
Some provocation for advanced readers
Re-exploring the foundations of electro-cardiology is always welcome. Worth diving deep into mysterious terminology non-specific ST/T changes. ST segment in the ECG corresponds to the most stressful period since it represents the active part of mechanical contraction. Curiously, it Includes the entire electrical (Repolarisation) & most parts of mechanical relaxation. The true onset of LV myocardial mechanical relaxation we can’t be sure, It happens somewhere in late phase 2. I think it’s so difficult to decode that timing. But, what we can presume is ST segment behavior in its distal half is less specific for both ischemic as well as hemodynamic stress
The electro-mechanical continuity within the ST segment is so intimate, and the demarcation point between them is invisible in many clinical situations. No surprise, we are largely in the dark about the true influence of the ST segment over T wave morphology and vice versa. (ie distal ST depression pulling down the T wave ) Though chronologically T must follow the S in timing, it would seem impossible for “T” to go back in time and pull the ST down. (If QRS can precede P in a junctional rhythm, why not T do the same for ST? ) I am not sure whether there is any timing involved in antegrade vs retrograde repolarisation across endo-epicardial repolarisation dissociation.Further, we know very well, myocardial scars cause fragmented depolarisation in QRS. Can anyone guess effect of these scars in repolarisation vectors? (Fragmented ST segment ?) I think it is worth pursuing this phenomenon. Let the young new age Sodipellares’ look into this.
Though the traditional rule of thumb, makes ST segment shifts more sinister, T-wave changes are largely benign, It is not an easy job to segregate benign from more serious forms of T-wave changes. Isolated new onset T inversion, can be an equally troublesome marker, especially mid-chest leads in the male population.
It is interesting to note, not every T wave Inversion is empowered to drag the ST segment down. We don’t know why. It is something to do with the curvature of the shoulder zone of phase 2 /3 of the action potential. In this context, ST dragging could be an important concept to explore.
Diastolic T wave stress
One more issue, which we are not yet clear is the timing of 2nd sound with reference to the T wave. It is a fact, a significant part of the T-wave will represent early diastolic hemodynamic stress* as well.