Myocardial infarction, a gross pathological entity renamed now as STEMI for clinical purposes, continues to be the most famous medical emergency that triggers a series of calls, right from 911 to the ER, that eventually ends up in CCU or a 24/7 cath lab. The heart, can’t wait for all these external responses when it is challenged with a vascular accident. The moment ATO occurs, two things happen. The endogenous fibrinolytic led by native tissue PA (Tpa) tries to get rid of the thrombotic plug by all its means. It succeeds in 15%. We call it spontaneous lysis or aborted MI. Many lives are lost in the remaining before they reach the hospital..
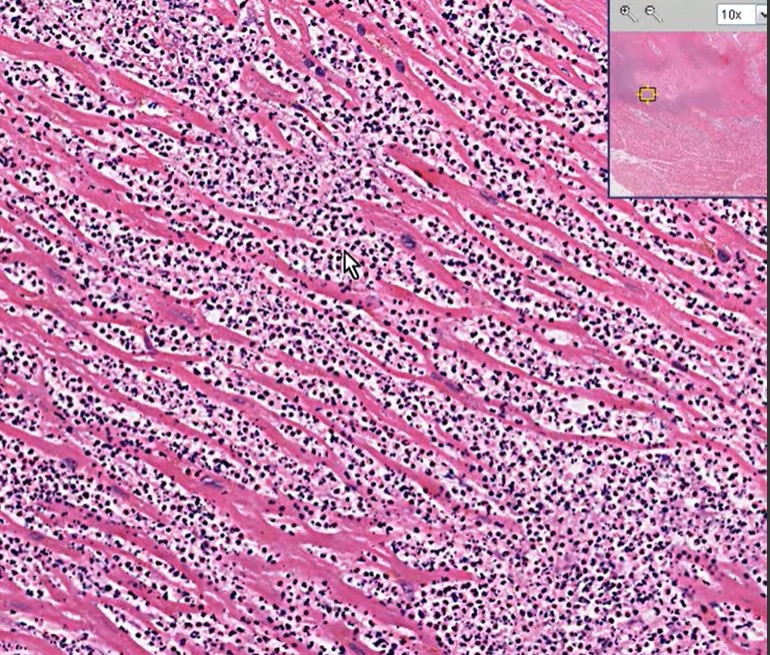
Meanwhile, the immune high-command deputes scores of neutrophils to the ground zero Fig 1) to supervise what is happening and try to heal the injury. It is worth, understanding, that activated WBCs, sort of convert myocardial infarction from a vascular event to inflammatory pathology, as the hour progress.
What do these neutrophils do?
It will be our ignorance, if we think, they simply crowd around the myocytes helplessly, that is hit by ischemia. They are sent with a specific purpose to protect and heal the myocardium. Very often it fails in its mission is a different story. These fighting neutrophils send a subtle signal of their presence with a mild increase in temperature during ACS ( Patel Prognostic usefulness of white blood cell count and temperature in acute myocardial infarction Am J Cardiol. 2005 Mar 1;95(5):614-8. )
Is neutrophil invasion good or bad for the myocardium?
These are pro-inflammatory cells. meant to promote healing and mitigate Injury. Interleukins and Leukotrienes do have a healing power as well. But, what happens, in reality, is, still a mystery. As of now, it is tempting to think, it does more damage than good. Its role changes over time.
Acute reperfusion Injury in Primary PCI
Neutrophils are quiet obedient cells generally, but once activated, their behavior can’t be predicted. It may start attacking the host cells, We know reperfusion injury is real and poorly understood. Delayed reperfusion, is well known to cause myocyte softening and lysis. What we know for sure is, primary PCI, induced accelerated flushing of these angry neutrophils is definitely related to no flow, microvascular plugging, and cardiogenic shock. (The fact that no-reflow and reperfusion injury is less of a problem in fibrinolysis demands introspection)
Diagnostic & prognostic value of neutrophilia
Neutrophilia is such a nonspecific response, faces ridicule for its clinical utility. But, In reality, it can be a worthy parameter, to time the age of the infarct (or even confirm* the ACS ) in otherwise equivocal clinical presentation.(Ref 2) More importantly, it provides prognostic information. One more potential use is Neutrophil count to guide the timing of surgery post-MI (as in VSR) A neutrophil count could help avoid the active phase of inflammation.
*I recall my surgical professor’s emphasis on leukocytosis to confirm acute appendicitis during the first clinical year.
Final message
There is no academic by-law, that forbids full-blown interventional cardiologists from having an affair with basic science. Unless the core science is irreversibly bonded to the bedside, we can never reap the true benefits of translational research. Hematological aspects in STEMI is one such underrated discipline. Also, we must encourage every postgraduate student in pathology/biochemistry/physiology to visit the CCUs or sit in the cath lab gallery more frequently. Watching the cardiology stalwarts plowing through the blocked coronary arteries, racing against time, is sure to kindle young minds, for a potential molecular breakthrough in cell survival and healing following myocardial hypoxia.
Reference
2. Thomson SP, Gibbons RJ, Smars PA, Suman VJ, Pierre RV, Santrach PJ, Jiang NS. Incremental value of the leukocyte differential and the rapid creatine kinase-MB isoenzyme for the early diagnosis of myocardial infarction. Annals of internal medicine. 1995;122:335–341. [PubMed] [Google Scholar]
3.. Cannon CP, McCabe CH, Wilcox RG, Bentley JH, Braunwald E. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. OPUS-TIMI 16 Investigators. Am J Cardiol. 2001;87:636–639. A610. [PubMed] [Google Scholar]
4. Bhatt DL, Chew DP, Lincoff AM, Simoons ML, Harrington RA, Ommen SR, Jia G, Topol EJ. Effect of revascularization on mortality associated with an elevated white blood cell count in acute coronary syndromes. Am J Cardiol. 2003;92:136–140. [PubMed] [Google Scholar]