Often general practitioners refer ECGs with abnormal resting ST/T wave patterns to cardiologists .
Following are few of them
- ST elevation
- ST depression
- T wave inversion
- Tall T waves
- A relatively uncommon finding is a flat ST segment , which is discussed here.
The commonest( benign) abnormality is T wave inversion in women and tall ST /T waves reflecting early repolarisation pattern in men. A flat ST segment is an occasional finding in general population.
ST segment is inscribed during the most important time of cardiac cycle.This is the period the ventricle is doing its prime function , namely ejecting the blood in systole .Hence it is subjected to maximum stress . During times of ischemia ST segment gets elevated or depressed depending upon the severity of ischemia. For the same reason , even subtle changes in this segment is frowned upon by cardiologists. Most of them would receive a EST.
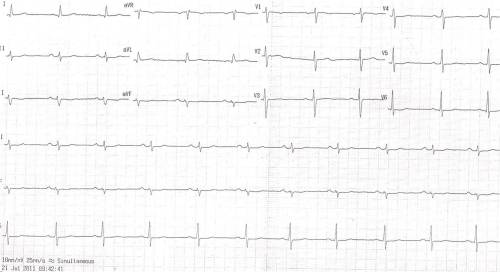
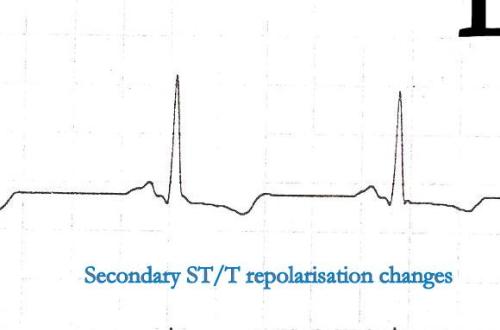
It is ironical to note , few normal people show almost silent electrical activity during this crucial phase of their ECG .ST segment is often a flat line in them . This is a ECG of a women referred as CAD. She was asymptomatic . Echocardiogram was normal . She was asked to do a EST.
The peculiar thing about T waves are , a 10 mm upright as well as 5 mm inverted T wave , both can be normal. So . there is no element of surprise to note absent T waves or a flat T wave to be called as normal .
* T waves are recorded when K+ efflux occur rapidly out of cells . Hypokalemia can be an important cause of flat T waves.
It is still a mystery to me why some people inscribe a tall T when potassium comes out of cell and an equal number (Esp women) record a down ward T wave for the same event ! I wish I get an answer to this lingering question from any of the readers !
Is a flat T wave represent a T wave in transition to become inverted T wave later ?
Possible .But we are not sure ! A static T wave is safer than a dynamic T wave .
Final message
Flat ST segment and absent T waves represent a same spectrum of ECG findings which are referred to as non specific ST segment changes in clinical practice .Generally , they have little clinical significance.* In our experience we have found , female patients, Anemia hypothyroidism are often associated with flat ST segments . If CAD is suspected exercise stress test should be done. Some believe a flat ST segment is more likely to result in EST positivity (Not necessarily true positive !)
* Non specific ST/T changes by itself is a huge topic. Ideally the term non specific ST /T changes should be avoided , as it primarily came into vogue to denote non ischemic ST segment (Still , other pathologies are very much possible) It is estimated there are about 50 causes for non specific ST/T changes , right from a benign situation like deep respirations , to significant myocardial disorders. However , it still makes good clinical sense for a general practitioner , to refer to a cardiologist , whenever ST segment deviates without any reason .